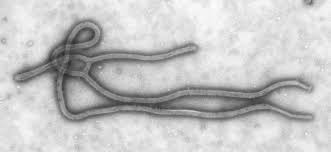
Two new rapid-acting Ebola vaccines have passed over hurdles: They have protected monkeys against the strain of the deadly virus that caused the recent outbreak in West Africa.
The vaccines worked against Ebola in one dose and appear to have no side effects. The test involved macaque monkeys because monkeys are closely related to humans. The monkeys that were vaccinated showed no signs of adverse reactions to the vaccines. When they were injected with the Ebola virus from the current outbreak in West Africa, they did not become ill. Unvaccinated monkeys injected with the Ebola virus died within a week.
Safety trials in people will be started early this summer. These two vaccines have been put on a fast track for human use because they appear to be so effective. They are being developed by Profectus BioSciences.
There are vaccines against Ebola that are already in human trials, but one of the ones being tested has had side effects including fever and muscle and joint pain. That vaccine is licensed to the Merck company and is an earlier version of the two vaccines being tested in monkeys. A different vaccine that is being tested in humans, licensed to GlaxoSmithKline, has had not serious side effects as yet.
Both vaccines are based on a recombinant version of the vesicular stomatitis virus (rVSV) that has been modified to produce a protein that is in the Zaire strain of Ebola virus. Both vaccines are attenuated, which means that the strain of rVSV virus they are based on is weakened.
The outbreak of Ebola virus in West African has killed more than 10,000 people since it started a year ago.
A report on the two vaccines was published in the journal Nature.
