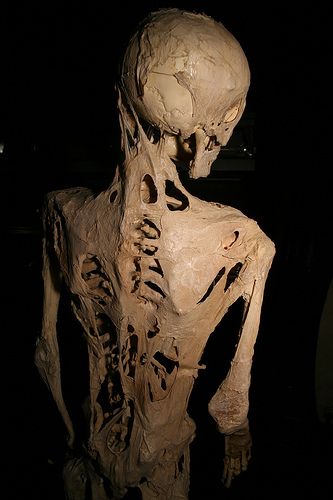
Researchers have found a possible treatment for a rare genetic condition that causes a person to become encased in bone. This treatment shuts down excessive bone growth in mice that have been genetically engineered to develop the disease, called fibrodysplasia ossificans progressiva (FOP).
Scientists from Regeneron Pharmaceuticals said they have found an antibody to a growth factor protein called Activin-A. This protein, which normally blocks bone growth, triggers hyperactive bone growth in patients with a genetic mutation for FOP.
The study was published in the journal Science Translational Medicine. The findings could eventually lead to a treatment for FOP.
In FOP, muscle and soft tissue are gradually turned into bone. Starting at around age 10, each time any soft tissue is injured or becomes inflamed, instead of the injury healing normally, soft tissue is replaced by bone. As the soft tissue turns to bone, patients are immobilized into one position and they eventually suffocate as their chest wall solidifies. The condition is very rare, with 800 patients globally, including 200 in the United States.
FOP is caused by mutations in a gene called ACVR1 which makes a receptor that controls bone growth. Researchers at Regeneron found that this malfunctioning receptor responds abnormally in the presence of Activin-A, which is often secreted by the immune system in response to injury and inflammation.
In people that do not have FOP, Activin-A blocks the receptor, stopping bone growth. But in people with FOP, Activin-A spurs bone growth.
To test their finding, researchers developed an antibody treatment designed to block Activin-A. In mice with a form of FOP, the antibody blocked the formation of excess bone.
This finding is exciting, but may not be able to translate directly into an FOP treatment for people. However, Regeneron says that it is conducting tests that could lead to clinical trials in people.
Another drug company, Clementia Pharmaceuticals Inc, is also studying FOP and is testing a compound called palovarotene, according to Reuters Health. Palovarotene interrupts the process of bone formation during disease flare-ups. It is in phase 2 trials with FOP patients.
