Researchers at the University of Illinois at Chicago are the first to identify the presence of a specific type of antibody, called anti-citrullinated protein autoantibodies, or ACPAs, in human tear fluid. They are also the first to demonstrate that patients with dry eye disease experienced reduced signs and symptoms of the condition in response to a new eye drop treatment --- made from pooled human antibodies -- that targets ACPAs.
The findings from their early stage clinical trial are reported in the journal The Ocular Surface.
Dry eye disease is caused by abnormalities in the tear fluid and results in dry areas over the cornea, the transparent outer layer of the eye, which can lead to disabling eye pain and sensitivity to light in severe cases.
"The burden of autoimmune dry eye is much greater than just having an occasional feeling of dryness," said Dr. Sandeep Jain, senior author of the study and UIC professor of ophthalmology and visual sciences at the College of Medicine. "It can severely compromise quality of life to the point of disability and can compromise a person's vision."
In previous research, Jain and his colleagues discovered that strands of DNA extrude from neutrophils, a type of white blood cell, to form webs on the surface of eyes affected by severe dry eye disease and cause inflammation.
In the new study, the researchers identified ACPAs as another cause of eye inflammation that also contributes to the development of these webs, which Jain calls "a vicious cycle of inflammation."
The new eye drops treat dry eye disease by knocking the immune system out of this cycle, at least partially.
The drops are formulated using pooled antibodies -- which are made from immune globulins processed from the donated blood of thousands of individuals, all containing varied types of antibodies -- that counteract the negative effects of ACPAs.
The phase I/II drug trial compared the antibody-based eye drops with eye drops without the antibodies.
"There are currently only two approved drugs to treat dry eye, and they don't work for everyone, especially those with severe disease, so having a new drug that can treat the disease by targeting a different mechanism, in this case, an autoimmunity, is very important," Jain said.
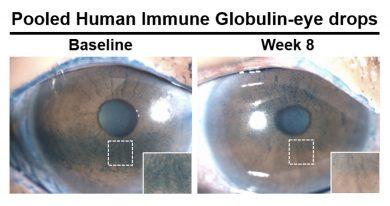
Twenty-seven participants with severe dry eye disease participated in the trial. The participants were randomized into two groups. One group was given eye drops made from pooled antibodies and instructed to administer one drop to each eye twice daily for eight weeks. The control group was given the same instructions with eye drops made without antibodies.
The researchers evaluated patients' symptoms through questionnaires and measured the extent of corneal damage and the amount of pro-inflammatory biomarkers on the surface of the eye before and for the duration of the study.
They found that people using antibody-based eye drops had a statistically significant and clinically meaningful reduction in corneal damage at eight weeks compared with the control group. Questionnaire scores related to symptoms also reflected significant improvement among patients using the new antibody-based eye drops when compared with the eye drops without antibodies. In the test group, the amount of pro-inflammatory biomarkers -- or dry areas -- also was reduced on the surface of the eye.
"Participants in the trial who used the drops with pooled antibodies reported less eye discomfort and their corneas were healthier," Jain said. "The data from this early clinical trial suggests that eye drops containing pooled antibodies may be safe and effective for treating dry eye disease, and we look forward to conducting larger randomized trials to definitively prove its efficacy."
