A new treatment for children with sickle cell anemia has been found to be so successful that a clinical trial of the drug was stopped. The results clearly showed that hydroxyurea therapy is safe and effective and that it can reduce the risk of stroke in these children.
The drug was being compared to monthly transfusions of red blood cells in 121 children with sickly cell anemia who were at high risk of stroke. Early results showed that the daily pill worked as well as transfusions in lowering the risk of stroke. The risk of stroke was determined in the patients by using ultrasound to measure the velocity of blood flow to the brain, a test called transcranial Doppler.
The randomized control trial was being conducted at 25 medical centers in the United States and Canada. The announcement that the study was being ended early came from the U.S. National Heart, Lung, and Blood Institute. Patients enrolled in the study were between age 4 and 16 who had sickle cell anemia and who had elevated risk of stroke. The first patient enrolled in the study in 2011.
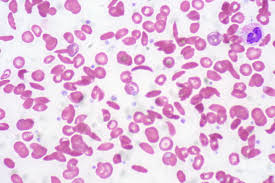
Sickle cell anemia, also called sickle cell disease, is a hereditary blood disorder in which red blood cells carry an abnormal hemoglobin molecule. Under certain circumstances, this molecule causes the red blood cells to go from their normal disc shape to a rigid sickle shape. The sickle-shaped cells cause clogs at branching points in blood vessels, which can result in a painful episode called a sickle cell crisis. If the cells clog up a blood vessel in the brain, the result can be a stroke.
One treatment for in children with sickle cell anemia has been regular transfusions of healthy red blood cells. Daily hydroxyurea has not been approved for use in children with sickle cell anemia as yet. Hydroxyurea was originally developed as an anticancer drug.



